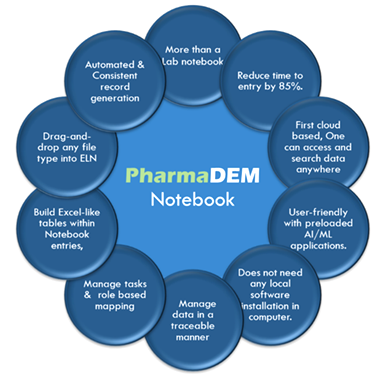
Pharma DEM ELN is a trusted, modern informatics solution that increases efficiency and productivity. We welcome the opportunity to partner with pharma companies so that scientists can focus on science rather than data formatting.
An Electronic Lab Notebook (ELN) is a software tool that in its most basic form replicates an interface much like a page in a paper lab notebook.
ELNs offer several advantages over traditional paper notebooks; they facilitate good data management practices, providing for data security, auditing, and collaboration.
Pharma R&D needs to unify and harmonize experimental data in the cloud/server, in order to fully capitalize on the power of AI and data science. In turn, AI and data science will uncover insights that will accelerate discovery and development of therapeutics that extend and enhance human life.