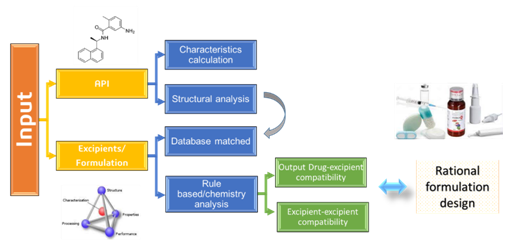
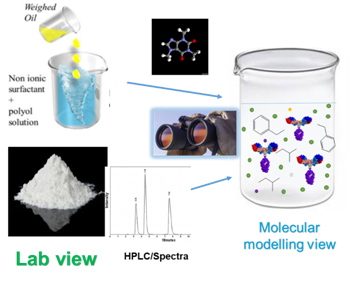
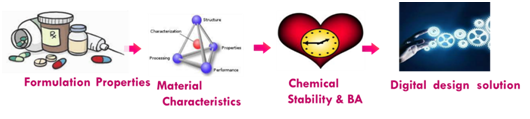
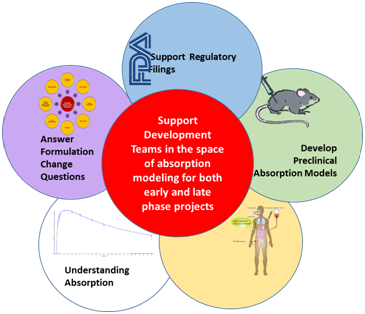
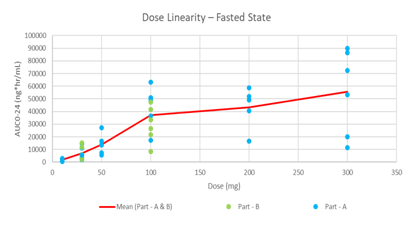
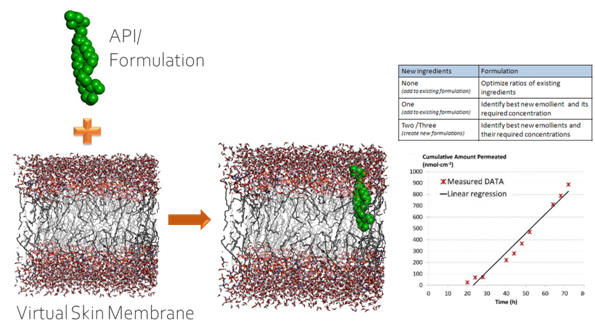
Advanced Drug Development Software: Transform Your Process
PharmaDEM's cutting-edge Drug Development Software is at your fingertips. Our innovative platform offers a range of powerful tools designed to streamline every stage of drug development, from formulation modeling to absorption simulation.